electron configuration of fe|fe2+ electron configuration condensed : Baguio Chlorine (Cl) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMD
YOUPORN to Twoja Baza Darmowych Filmików PORNO XXX. OGLĄDAJ najlepszy sex MAŁOLATÓW w sieci! Zobacz filmiki z sexy panienkami nago w akcji!
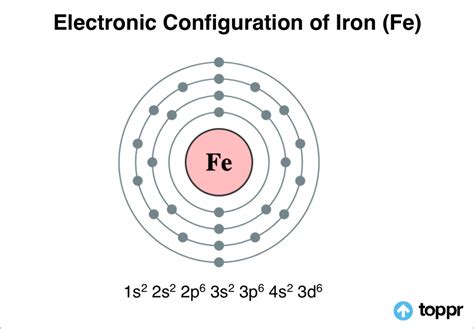
electron configuration of fe,The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write .
In order to write the Calcium electron configuration we first need to know the .
Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .
In order to write the Mg electron configuration we first need to know the .Chlorine (Cl) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMD
Phosphorus (P) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMD
In order to write the Argon electron configuration we first need to know the .How to Write the Electron Configuration for Oxygen. Oxygen is the eighth element .
Mar 23, 2023
Learn the electronic configuration of iron (Fe) with 26 electrons, its oxidation states, allotropic forms and applications. Find out how iron forms bonds, why it has two valencies and how to write its configuration.
Learn how to write the electron configuration of iron (Fe) using the Bohr model of orbits and the Aufbau principle of orbitals. Compare the differences and similar.
electron configuration of fe fe2+ electron configuration condensed The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, . Learn how to determine the ground state electron configuration of iron (Fe) using the Aufbau diagram and the periodic table. See the answer and explanation from two different sources.Learn how to write the electronic configuration of iron (Fe) and its oxidation states (+2 and +3) with examples and diagrams. Also, find out the valency and applications of iron in . The electronic configuration of \[Fe^{2+} is - 1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 3d^{6},\] while that of \[Fe^{3+} is 1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 3d^{5}\]. . By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the .
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that .

Electron Configuration for Fe. The iron electron configuration is one of the other significant areas of study for scholars of chemistry. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, the Iron electron configuration is .fe2+ electron configuration condensed The Electronic Configuration of Iron. A chemical element with an atomic number 26 and a symbol Fe represents it, Iron is a very common element that is found. Unlike other elements, iron exists at the oxidation states of -2 to +6. Elementary iron occurs in a low-oxygen environment in spite of being reactive to water and oxygen.

Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as .electron configuration of feElectron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. . What is the electronic configuration of #Fe^(3+)# ion? Question #19968. Question #3c8ff. What is the electronic configuration of magnesium? Question #3df7e. Question #32589.
Start with the electron configuration of the neutral atom. It is sufficient to use the noble gas configuration. Fe: [Ar] 4s 2 3d 6. Then, remove (or add) electrons to reflect the oxidation state of the metal. Remember to remove electrons from the orbital with the highest principal quantum number first! Fe(III) has lost 3 electrons relative to .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon . Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe 2+: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 = 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. (e). Sm: 1s 2 2s 2 2p 6 . To know the abbreviated fe3+ electron configuration first of all we must know the abbreviated electronic configuration of fe atom. The abbreviated fe electron configuration is [Ar] 3d6 4s2. By counting the electrons from 1s to 3p orbital we find that there is a total number of 18 electrons and we replace it by writing in the form of [Ar].To check the answer, verify that the subscripts add up to the atomic number. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83).
electron configuration of fe|fe2+ electron configuration condensed
PH0 · full electron configuration cu
PH1 · fe2+ electron configuration condensed
PH2 · fe +2 def how many electrons added
PH3 · electron configuration periodic table
PH4 · electron configuration for every element
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · complete electron configuration
PH8 · Iba pa